[ad_1]

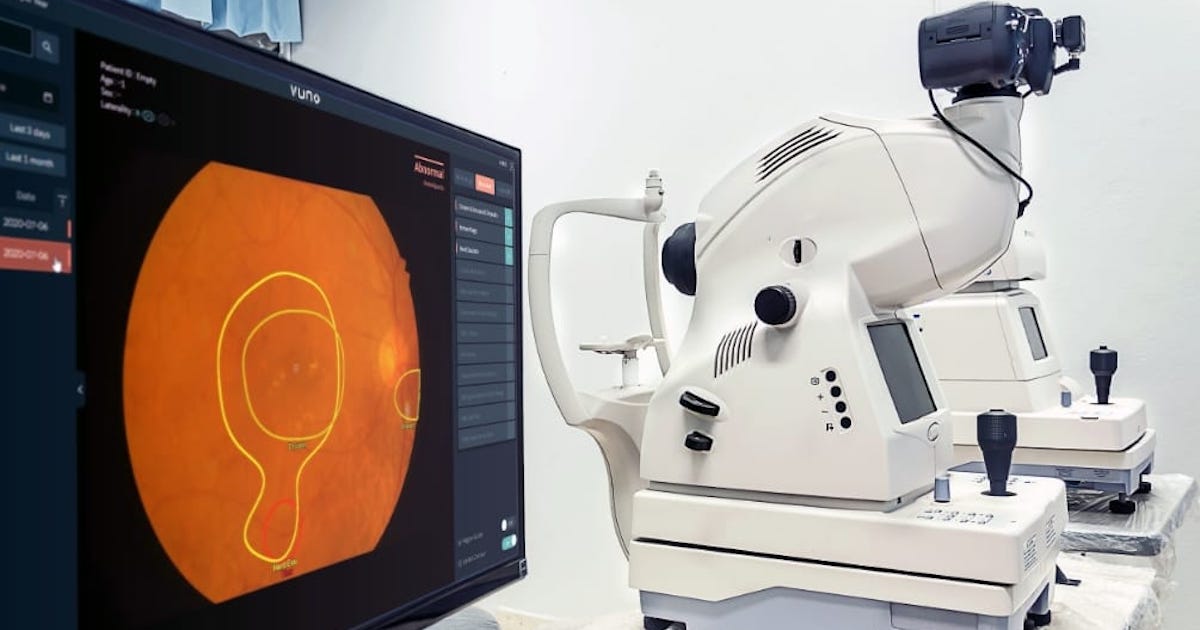
Singapore clears VUNO’s AI software for fundus analysis
South Korean medical AI firm VUNO has recently obtained a medical device certification from Singapore’s Health Sciences Authority for its AI-based fundus analysis software.
The VUNO Med-Fundus AI analyses images of the fundus, which is the back part of the eye, to provide findings necessary for the diagnosis of retinal diseases. It can detect the location of lesions indicative of diseases, such as diabetic retinopathy, macular disease and glaucoma, within seconds.
Through this certification, VUNO can tap into the growing medical devices market in Singapore, which is projected to reach $1 billion in value in 2024, growing at an 8.7% CAGR from 2019.
Taiwan-based Point Robotics gets 510(k) for its surgical robot system
Point Robotics, maker of surgical robots in Taiwan, has received the US Food and Drug Administration’s 510(k) clearance for its integrated robotic-assisted surgical system for spinal fusion surgeries.
The POINT Kinguide Robotic-Assisted Surgical System combines image-guided navigation and a hand-held drilling feature to streamline procedural tasks with precision, stability, and reproducibility of robot motion.
What sets it apart from similar systems, according to the company, is the parallel manipulator mechanism that enables it to expand indications for more complicated herniated disc decompression surgery.
Following the US clearance, Point Robotics is preparing for CE marking in Europe and registration in China, expanding its presence in international markets.
“We aim to promote availability and affordability of robot’s adoption for spinal surgery unaddressed by today’s technology to treat more patients who contracted a spectrum of spinal diseases,” said CEO SC Juang.
Mental health startup Lissun receives $1M in pre-seed funding
Indian mental health platform Lissun has raised $1 million in a pre-seed funding round led by IvyCap Ventures. The round was also participated by We Founder Circle, Supermorpheous and other marquee angel investors.
The startup employs a unique “B2H2C” approach – it reaches consumers through its partnerships with healthcare institutions – in offering its full-stack mental health solution. It has tie-ups with over 70 healthcare organisations in 17 cities in India.
Based on a press statement, Lissun’s technology is applied to high-stress use cases in six healthcare categories, such as infertility, rehabilitation, nephrology, and oncology, among others. “The very fact that mental and emotional issues can be an underlying problem in many medical cases is what we have identified and are working on proactively,” explained co-founder Dr Krishna Veer Singh.
Through its fresh investment, Lissun aims to strengthen its technology’s backbone and further develop it to provide a seamless user experience. Moreover, it plans to expand to 25 cities, as well as cover five more therapeutic categories.
India to repurpose COVID-19 apps
The Indian government plans to repurpose its two main mobile applications for tracking COVID-19 cases in the country.
A news report cited Dr Ram Sewak Sharma, CEO of the National Health Authority, as saying that they plan to reuse the contact tracing app Aarogya Setu as a national health app while the Covid Vaccine Intelligent Network (CoWIN) platform will be repurposed for the national immunisation programme and a health management information system for small doctors.
[ad_2]
Source link





No comment yet, add your voice below!